Abstract
Background:
Primary immune thrombocytopenia (ITP) is the most common autoimmune hemorrhagic disorder characterized by decreased platelet count. increased risk of bleeding, and poor quality of life. Only about 70% patients response to first-line treatments, some patients are still refractory or relapsed after combined therapies, therefore it is necessary to explore new therapeutic targets. Bruton's tytosine kinase (BTK) is a non-receptor tyrosine kinase of Tec family, which is widely expressed in hematopoietic cells including B cells, monocytes/macrophages, and others. BTK participates in a variety of signaling pathways of innate and adaptive immunity, and plays an important role in cell survival and maturation. Platelet destruction mediated by anti-platelet glycoprotein antibodies is considered to be the main cause of ITP. B cells differentiate into plasma cells and produce autoantibodies due to the intolerance to autoantigens, which are important effectors in the pathogenesis of ITP. We speculated that inhibition of BTK may reduce platelet destruction by inhibiting B cell activation and autoantibody production. Orelabrutinib is a new generation of BTK inhibitor which has been used in hematological malignancies, this is the first study to explore the mechanisms of BTK inhibitor in the treatment of ITP.
Methods:
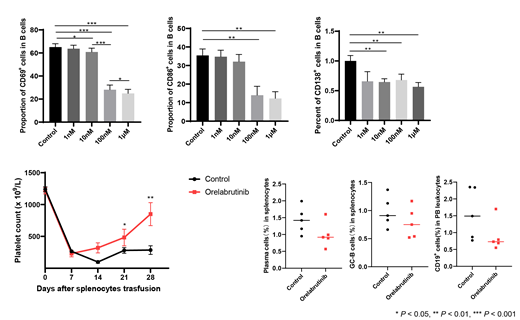
The concentrations of orelabrutinib were set at 1 nM, 10 nM, 100 nM and 1 μM in the in vitro study. Peripheral blood mononuclear cells (PBMCs) were isolated from active ITP patients and healthy controls and cultured for 72 hours, the apoptosis rate of PBMCs in each group was measured by Annexin V/PI double staining. CD19 + B cells of ITP patients were sorted by magnetic beads and stimulated with anti-human IgM to evaluate the activation of B-cell receptor (BCR) pathway and differentiation of plasma cells, respectively. Further, we transfused the splenocytes of immunized CD61-KO mice (C57BL/6) into the severe combined immunodeficient (SCID) mice to establish the active ITP murine models. Orelabrutinib was administered intragastrically at 10mg/kg, once a day. The control group was treated with 0.5% CMC at the same volume and frequency. Platelet count was measured weekly, the peripheral blood was collected and the B cell subsets in spleen were detected by flow cytometry at days 28 after splenocyte transfusion.
Results:
The proportion of early apoptotic cells (Annexin V +PI -) in PBMCs from both ITP patients and healthy controls was increased by orelabrutinib at 1μM,but there was no statistical difference. Orelabrutinib significantly inhibited the expression of CD69 in a dose-dependent manner at the concentrations of 10nM, 100nM and 1μM. Another early activation marker of BCR signaling pathway, CD86, was also found to be inhibited by orelabrutinib at 100nM and 1μM. The number of CD138 + plasma cells was decreased after treated with orelabrutinib at 10nM, 100nM and 1μM without dose-dependent manner. In the murine models, mice administered with orelabrutinib had significantly higher platelet count than the control mice at days 7, 14, 28 after splenocyte transfusion. The frequency of total B cells in peripheral blood leukocytes, the proportion of GL-7 + germinal center B cells and plasma cells in splenocytes were all determined to be lower in mice treated with orelabrutinib than the control group, though did not reach the statistical significance.
Conculsion:
Orelabrutinib could effectively suppress the activation and differentiation of B cells invitro and invivo, thus alleviate the thrombocytopenia in active ITP murine models. It could be the new treatment strategy for refractory/relapsed ITP patients.
No relevant conflicts of interest to declare.